barostim neo system
This pacemaker-like device is designed to electrically activate the baroreflex the bodys main cardiovascular reflex signaling the brain to regulate cardiovascular function. 32 to the medical management arm and 40 to the device arm 38 implanted 2 withdrawn.

Investigational Device For Heart Failure Patients Stimulates Cells In Arteries To Improve Function Youtube
A prospective randomized study describing the safety and efficacy of the BAROSTIM NEO System in heart failure subjects with left ventricular ejection fraction equal to or less than 35 percent.
. During long-term follow-up all participants are required to have at least one annual visit. The Barostim neo system is CE marked for the treatment of heart failure with reduced ejection fraction. The brain then sends the necessary signals to the blood vessels and heart to reduce heart failure symptoms.
It is also CE marked for the treatment of resistant hypertension. Food and Drug Administration FDA-approved device that uses a novel mechanism to improve heart function. BAROSTIM NEO is a US.
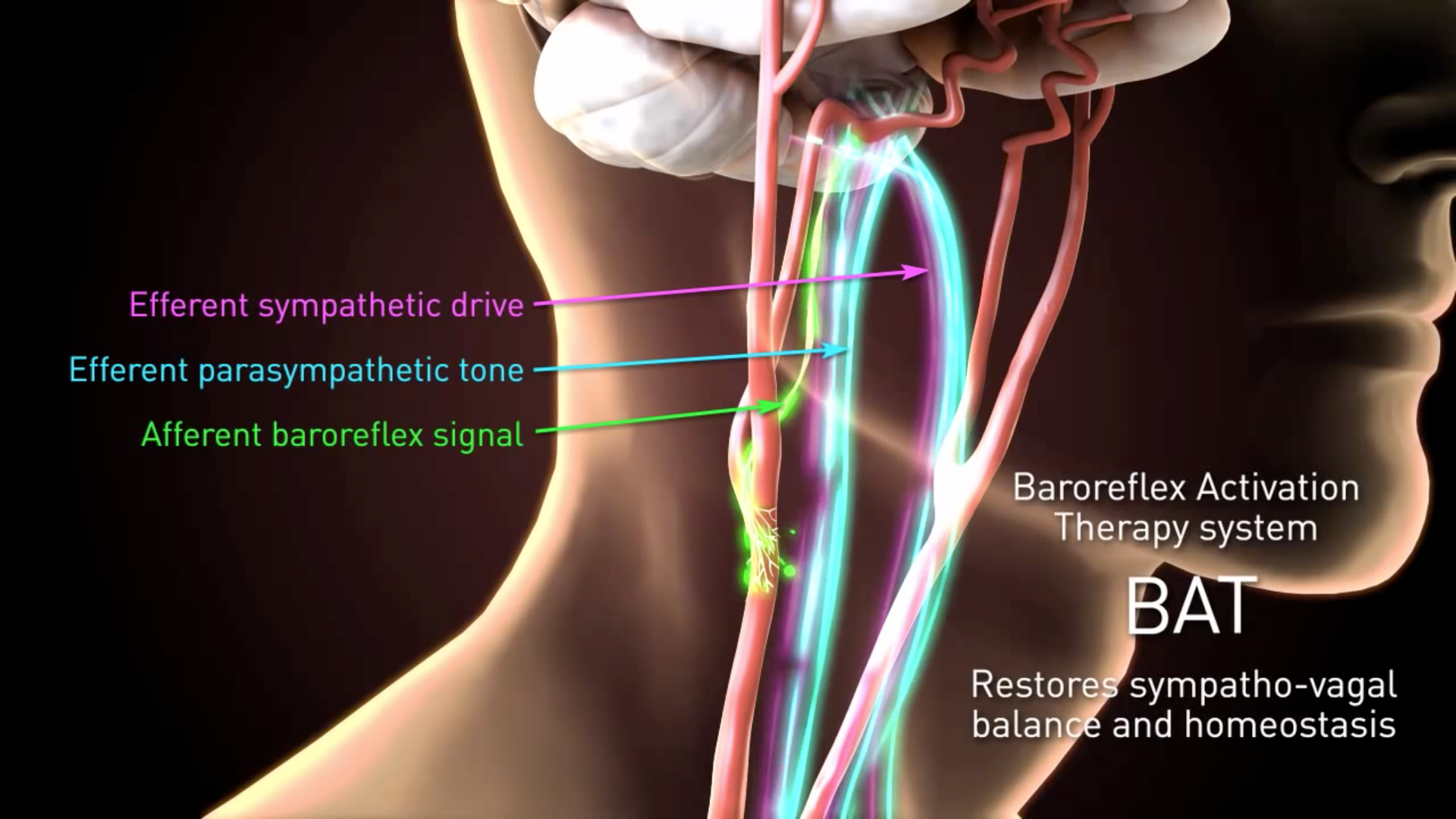
Barostim neo is a second generation device that uses CVRx-patented technology that is designed to trigger the bodys own natural blood flow regulation system to treat patients suffering from. Barostim Neo - Baroreflex Activation Therapy for Heart Failure Study Title. The mechanism of action is electrical stimulation of the baroreceptors which does not alter or destroy the structure of the baroreflex.
Barostim is a novel Congestive Heart Failure CHF treatment that uses the power of the brain to improve symptoms like breathlessness fatigue and swelling. Barostim Neo is a Food and Drug Administration-approved device which uses a novel mechanism to improve cardiovascular function. Barostim was estimated to be cost-effective compared with optimal medical treatment with an incremental cost-effectiveness ratio of 7 797QALY.
The Barostim neo is an electrical stimulator with a lead going up to the carotid artery where it excites baroreceptors that in turn regulate. Barostim Neo - Baroreflex Activation Therapy for Heart Failure. Seventy two subjects were randomized.
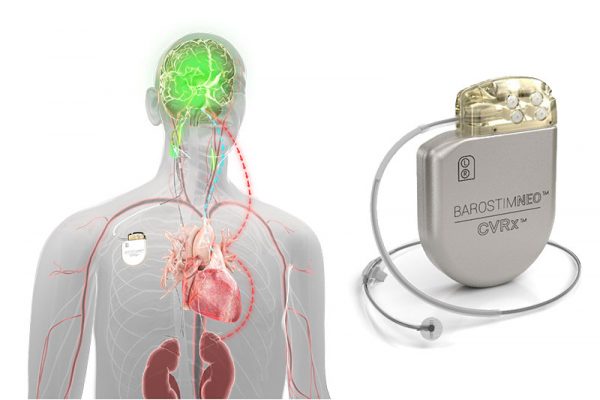
The minimally-invasive neosystem uses CVRx patented Barostim Therapy technology to trigger the bodys own natural systems by electrically activating the carotid baroreceptors the bodys natural cardiovascular regulation sensors. The implanted Barostim Neo System sends electrical impulses to cells in the neck called baroreceptors. The system received CE mark from the National Standards Authority of Ireland NSAI in September 2014 to treat heart failure patients with an ejection fraction less than or equal to 35.
The therapy can be turned OFF by simply pressing a button to easily observe the difference Barostim neohas on blood pressure and other hemodynamic parameters. Barostim neois a subcutaneous reversible treatment. In a single-arm open-label study Hoppe and associates 2012 evaluated the effectiveness of the Barostim neo system a second-generation system for delivering BAT.
Barostim is an FDA approved implantable device to treat people with CHF and a low ejection fraction systolic heart failure who do not qualify for Cardiac Resynchronization Therapy CRT. The BAROSTIM NEO System is marketed in the European Union and countries recognizing the CE marking for the treatment of heart failure since August 8 2014. The BAROSTIM NEO System Premarket Approval P180050 is a Class III carotid sinus stimulator an implantable medical device that delivers electrical signals to the bodys pressure sensors to.
The BAROSTIM NEO System is designed to electrically activate the carotid baroreceptors the bodys natural cardiovascular regulation sensors. Subjects were patients with resistant hypertension SBP greater than or equal to 140 mm Hg despite treatment with 3 or more medications including 1 ore more diuretic. The Neo Randomized Heart Failure Study is a prospective randomized study describing the safety and efficacy of the BAROSTIM NEO System in the heart failure participants with a left ventricular ejection fraction 35.
These receptors in the neck sense the blood that is flowing through the carotid arteries. Barostim Neo is a neuromodulation system developed by CVRx for the treatment of heart failure and hypertension. Late last week the US Food and Drug Administration granted premarket approval to the Barostim Neo baroreflex activation therapy device CVRx which is used to improve symptoms in patients with heart failure with reduced ejection fraction who are not eligible for cardiac resynchronization therapy CRT.
Back to Approved IDE Studies. As a result information is sent to the brain. This pacemaker-like device is designed to electrically activate the baroreflex the bodys main cardiovascular reflex which signals the brain to regulate heart function.
Austria Czech Republic France Germany Italy Lebanon. Research Statistics Data. This one-of-a-kind therapy reduces the workload of the heart by decreasing arterial resistance thereby improving the hearts ability to pump blood to the tissues.
The neosystem is the CVRx next generation system for improving cardiovascular function. In the model Barostim reduced over a lifetime the rates of myocardial infarction by 19 stroke by 35 heart failure by 12 and end-stage renal disease by 23. The Barostim neo system is for patients having an ejection fraction less than or equal to 35 and a New York Heart Failure Classification of III without restriction on QRS duration concomitant medical device treatment or presence of atrial fibrillation.
The following is a listing of countries where BAROSTIM NEO System has been marketed for the treatment of heart failure. Project Type Neuromodulation system Developer CVRx. The Barostim Neo System is indicated for patients who have a regular heart rhythm are not candidates for cardiac resynchronization therapy and have a.
According to the FDA the CVRx Barostim Neo System is indicated to be used in patients with a regular heart rhythm are not suitable for cardiac resynchronization therapy and have a left ventricular ejection fraction of under or equal to 35 which is considered less than the normal ejection fraction of 55-75.

Barostim Neo Electrical Stimulator Approved For Heart Failure In Europe Video Medgadget

Baroreflex Activatie Therapie Ppt Download
Cvrx S Barostim Neo Gets Ce Mark For Use With Mris Massdevice

Barostim Neo Neuromodulation Implantable System Usa

Baroreflex Activation Therapy Study In China 2022 Wiki English

Barostim Neo Electrical Stimulator Approved For Heart Failure In Europe Video Medgadget

Fda Approves Device To Treat Patients With Heart Failure Wjar

Interventional Treatment For Heart Failure Receives Fda Approval Biba Medtech Insights

Barostim Neo Neuromodulation Implantable System Usa

Barostim Neo Neuromodulation Implantable System Usa
About Us Cardiovascular Interventions

Cvrx Barostim Neo Now Cleared For Mri Use In Europe Medaxs

Baroreflex Activation Therapy By The Rheos System And The Barostim Download Scientific Diagram

First Rheos And Second Barostim Neo Generation Baroreflex Download Scientific Diagram
Barostim Neo 1 Radcliffe Cardiology
First Implant Made For Barostim Neo Device To Treat Hypertension Daic

Baroreflex Activation Therapy Barostim Neo System Tctmd Com

Fda Approves Device To Treat Patients With Heart Failure Wjar

Comments
Post a Comment